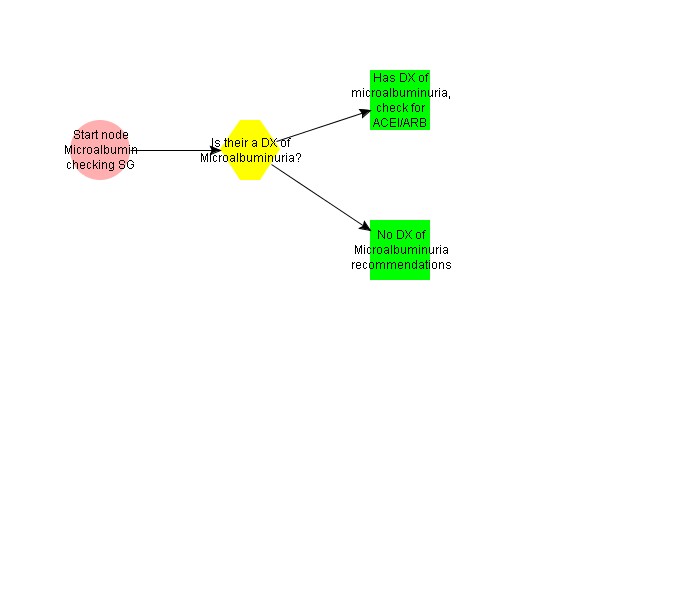
This subguideline handles elevated microalbumin levels and makes appropriate recommendations
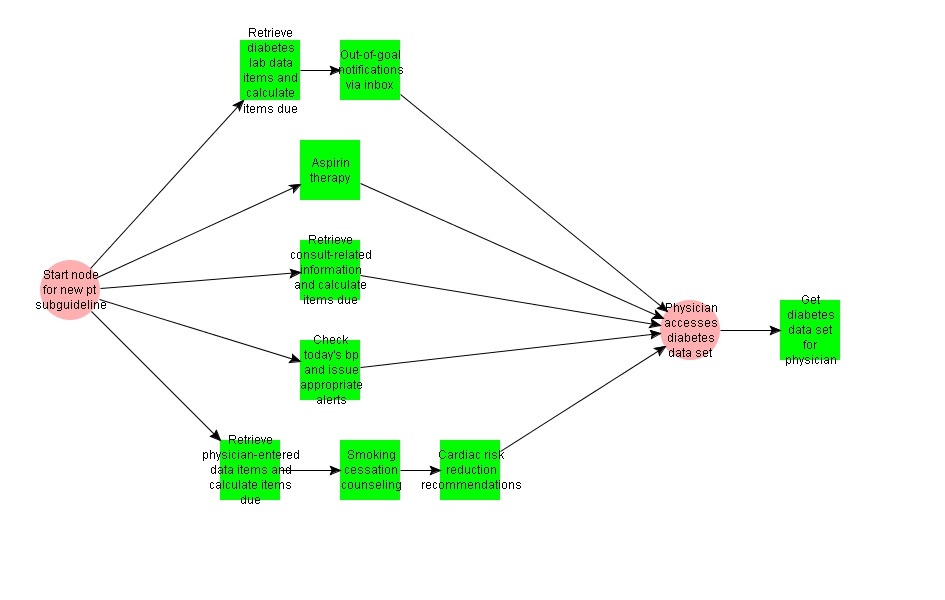
This Recommendation Set encompasses a primary care clinic visit by a diabetic patient.

This activity graph checks the patient record for initial data values and calculates what data is needed at this time.

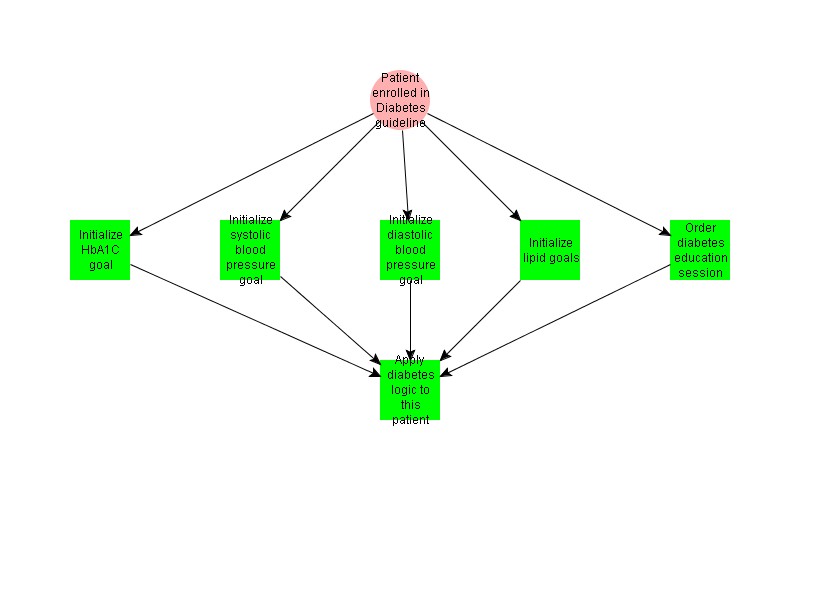
When a patient is first enrolled in the Diabetes guideline, the default goal values for clinical parameters are set. The user receives a prompt to enter diabetes onset date, enter type of diabetes (I or II), and order a Diabetes Education session.
The guideline checks whether the BP has been entered today. If not, the clinician is notified. If so, if the BP is below goal than this is noted. If the BP is above goal, then the problem list is checked for Hypertension. If HTN, then the HTN recommendation subguideline is triggered. If the pt does not have HTN, the Elevated BP subguideline is triggered.

This activity graph includes logic for population management. The outcome will be a single saved order session containing all needed orders and labelled as originating from the SAGE Diabetes population-based management guideline.

Diabetes logic, the same as that applied during a diabetes clinic visit, is applied to this patient.
These recommendations follow the 2004 ADA "Aspirin Therapy in Diabetes" guideline. If the patient has no contraindications and is > 40 yo, or > 20 yo with CVD, HTN, tobacco use, dyslipidemia, albuminuria, FHx of CVD, or Hx of MI, vascular bypass procedure, stroke, TIA, PVD, claudication, or angina, aspirin therapy should be considered.

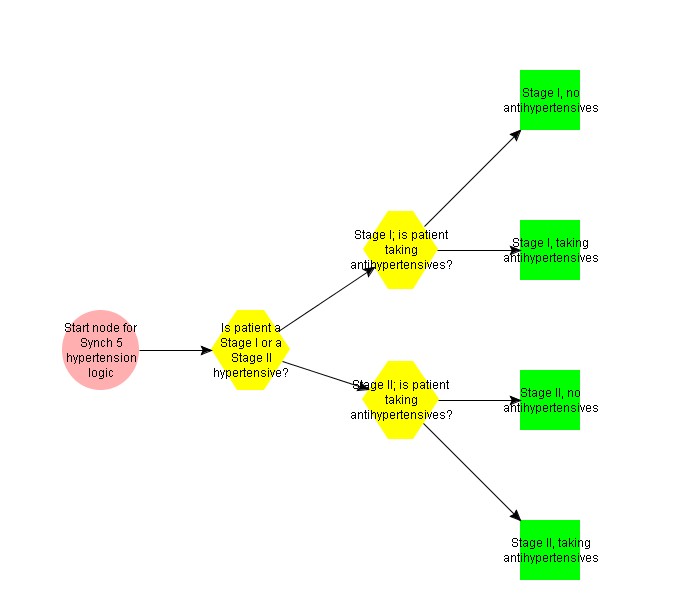
Check for Stage I or Stage II hypertension, and recommend one or two therapies as appropriate.
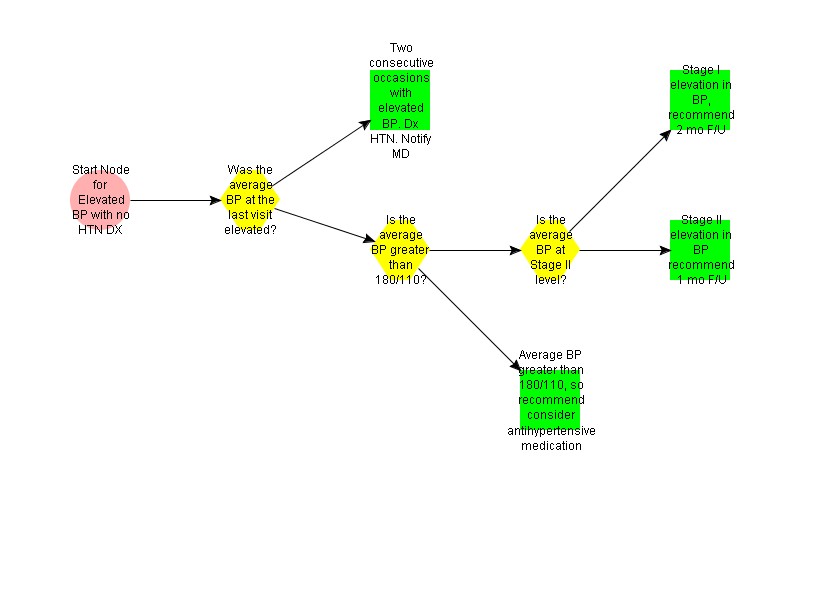
This subguideline makes recommendations for patients with elevated blood pressure, but no current diagnosis of HTN.
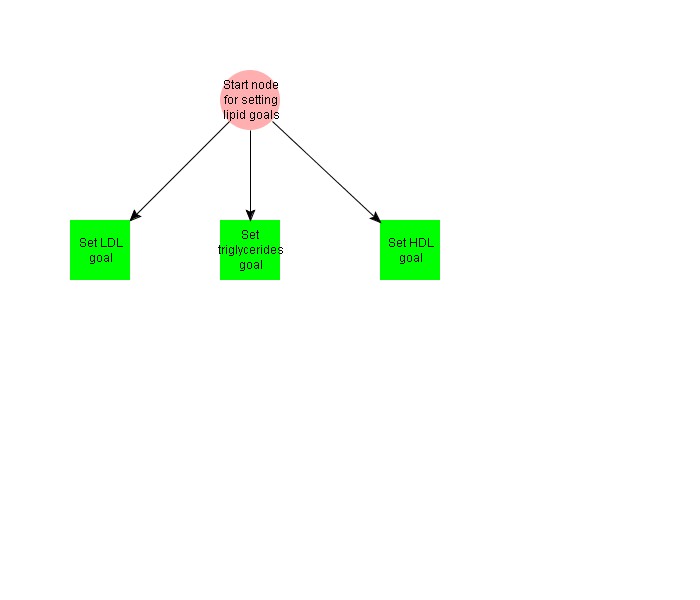
This decision map sets lipid goals to levels appropriate for a diabetic patient, with the precondition of Diabetes Mellitus on the problem list.
This subguideline performs checking for lipid parameters in adult and pediatric patients.

| Condition | is | to | source |
| presence of Observation Patient currently pregnant (finding) [SNOMED CT] | Absolute_contraindication | ACE Inhibitor Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex, NDF-RT, JNC VI )
|
| presence of Problem Angiotensin-converting-enzyme inhibitor allergy (disorder) [SNOMED CT] | Absolute_contraindication | ACE Inhibitor Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex, NDF-RT, Epocrates )
|
| presence of Problem Hyperkalemia (disorder) [SNOMED CT] | Relative_contraindication | ACE Inhibitor Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex )
|
| presence of Problem Angioedema (disorder) [SNOMED CT] | Absolute_contraindication | ACE Inhibitor Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( NDF-RT )
|
| presence of Problem Aortic valve stenosis (disorder) [SNOMED CT] | Relative_contraindication | ACE Inhibitor Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex )
|
| presence of Problem Renal artery stenosis (disorder) [SNOMED CT] | Relative_contraindication | ACE Inhibitor Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex, NDF-RT )
|
| presence of Problem Aldosterone antagonists allergy (disorder) [SNOMED CT] | Absolute_contraindication | Aldosterone Receptor Blocker Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex, NDF-RT, Epocrates )
|
| presence of Problem Type 2 diabetes mellitus with persistent microalbuminuria (disorder) [SNOMED CT] | Absolute_contraindication | Aldosterone Receptor Blocker Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex, Epocrates )
|
| presence of Problem Hyperkalemia (disorder) [SNOMED CT] | Absolute_contraindication | Aldosterone Receptor Blocker Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex, NDF-RT, Epocrates )
|
| presence of Problem Renal impairment (disorder) [SNOMED CT] | Relative_contraindication | Aldosterone Receptor Blocker Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex, Epocrates )
|
| presence of Problem Acidosis (disorder) [SNOMED CT] | Relative_contraindication | Aldosterone Receptor Blocker Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex )
|
| presence of Problem Hepatic failure (disorder) [SNOMED CT] | Relative_contraindication | Aldosterone Receptor Blocker Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex )
|
| presence of Problem Alpha-adrenoceptor blocking drug allergy (disorder) [SNOMED CT] | Absolute_contraindication | Alpha 1 Blocker Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex, NDF-RT, Epocrates )
|
| presence of Problem Disorder of liver (disorder) [SNOMED CT] | Relative_contraindication | Alpha 1 Blocker Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex )
|
| presence of Problem Acute cerebrovascular insufficiency (disorder) [SNOMED CT] | Relative_contraindication | Alpha 1 Blocker Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex )
|
| presence of Problem Angioedema (disorder) [SNOMED CT] | Relative_contraindication | Angiotensin II Antagonist Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex, NDF-RT )
|
| presence of Problem Aortic valve stenosis (disorder) [SNOMED CT] | Relative_contraindication | Angiotensin II Antagonist Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex )
|
| presence of Problem Mitral valve stenosis (disorder) [SNOMED CT] | Relative_contraindication | Angiotensin II Antagonist Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex )
|
| presence of Problem Renal impairment (disorder) [SNOMED CT] | Relative_contraindication | Angiotensin II Antagonist Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex )
|
| presence of Problem Renal artery stenosis (disorder) [SNOMED CT] | Relative_contraindication | Angiotensin II Antagonist Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( NDF-RT )
|
| presence of Observation Patient currently pregnant (finding) [SNOMED CT] | Absolute_contraindication | Angiotensin II Antagonist Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex, NDF-RT, Epocrates )
|
| presence of Problem First degree atrioventricular block (disorder) [SNOMED CT] | Relative_contraindication | Nonselective Beta-Blocker Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex )
|
| presence of Problem Chronic obstructive lung disease (disorder) [SNOMED CT] | Relative_contraindication | Nonselective Beta-Blocker Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex )
|
| presence of Problem Cardiogenic shock (disorder) [SNOMED CT] | Absolute_contraindication | Nonselective Beta-Blocker Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex, NDF-RT, Epocrates )
|
| presence of Problem Non-cardioselective beta-blocker allergy (disorder) [SNOMED CT] | Absolute_contraindication | Nonselective Beta-Blocker Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex, NDF-RT, Epocrates )
|
| presence of Problem Congestive heart failure (disorder) [SNOMED CT] | Relative_contraindication | Nonselective Beta-Blocker Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( NDF-RT )
|
| presence of Problem Incomplete atrioventricular block (disorder) [SNOMED CT] | Absolute_contraindication | Nonselective Beta-Blocker Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex, Epocrates )
|
| presence of Problem Complete atrioventricular block (disorder) [SNOMED CT] | Absolute_contraindication | Nonselective Beta-Blocker Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex, Epocrates )
|
| presence of Problem Severe sinus bradycardia (disorder) [SNOMED CT] | Absolute_contraindication | Nonselective Beta-Blocker Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex )
|
| presence of Problem Acebutolol allergy (disorder) [SNOMED CT] | Absolute_contraindication | Acebutolol (product) [SNOMED CT] |
Supplemental_Material ( Micromedex, NDF-RT, Epocrates )
|
| presence of Problem Incomplete atrioventricular block (disorder) [SNOMED CT] | Absolute_contraindication | Beta-blockers with Intrinsic Sympathomimetic Activity Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex )
|
| presence of Problem Complete atrioventricular block (disorder) [SNOMED CT] | Absolute_contraindication | Beta-blockers with Intrinsic Sympathomimetic Activity Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex )
|
| presence of Problem Severe sinus bradycardia (disorder) [SNOMED CT] | Absolute_contraindication | Beta-blockers with Intrinsic Sympathomimetic Activity Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex )
|
| presence of Problem Cardiogenic shock (disorder) [SNOMED CT] | Absolute_contraindication | Beta-blockers with Intrinsic Sympathomimetic Activity Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex, NDF-RT )
|
| presence of Problem Congestive heart failure (disorder) [SNOMED CT] | Relative_contraindication | Beta-blockers with Intrinsic Sympathomimetic Activity Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex, NDF-RT, Epocrates )
|
| presence of Problem Asthma (disorder) [SNOMED CT] | Absolute_contraindication | Beta-blockers with Intrinsic Sympathomimetic Activity Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex, NDF-RT )
|
| presence of Problem Chronic obstructive lung disease (disorder) [SNOMED CT] | Relative_contraindication | Beta-blockers with Intrinsic Sympathomimetic Activity Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex )
|
| presence of Problem Cardiogenic shock (disorder) [SNOMED CT] | Absolute_contraindication | Cardioselective Beta-Blocker Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex, NDF-RT )
|
| presence of Problem Incomplete atrioventricular block (disorder) [SNOMED CT] | Absolute_contraindication | Cardioselective Beta-Blocker Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex, NDF-RT )
|
| presence of Problem Complete atrioventricular block (disorder) [SNOMED CT] | Absolute_contraindication | Cardioselective Beta-Blocker Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex, NDF-RT )
|
| presence of Problem Severe sinus bradycardia (disorder) [SNOMED CT] | Absolute_contraindication | Cardioselective Beta-Blocker Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex )
|
| presence of Problem Sick sinus syndrome (disorder) [SNOMED CT] | Absolute_contraindication | Cardioselective Beta-Blocker Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex, NDF-RT )
|
| presence of Problem Sick sinus syndrome (disorder) [SNOMED CT] | Absolute_contraindication | Combined Alpha- and Beta-Blocker Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex, NDF-RT, Epocrates )
|
| presence of Problem Severe sinus bradycardia (disorder) [SNOMED CT] | Absolute_contraindication | Combined Alpha- and Beta-Blocker Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex, NDF-RT, Epocrates )
|
| presence of Problem Cardiogenic shock (disorder) [SNOMED CT] | Absolute_contraindication | Combined Alpha- and Beta-Blocker Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex, NDF-RT, Epocrates )
|
| presence of Problem Asthma (disorder) [SNOMED CT] | Absolute_contraindication | Combined Alpha- and Beta-Blocker Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex, Epocrates )
|
| presence of Problem Incomplete atrioventricular block (disorder) [SNOMED CT] | Absolute_contraindication | Combined Alpha- and Beta-Blocker Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex, Epocrates )
|
| presence of Problem Complete atrioventricular block (disorder) [SNOMED CT] | Absolute_contraindication | Combined Alpha- and Beta-Blocker Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex, Epocrates )
|
| presence of Problem Diltiazem allergy (disorder) [SNOMED CT] | Absolute_contraindication | Diltiazem (product) [SNOMED CT] |
Supplemental_Material ( Micromedex, NDF-RT, Epocrates )
|
| presence of Problem Cardiogenic shock (disorder) [SNOMED CT] | Absolute_contraindication | Calcium Channel blocker Non-Dihydropyridine Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex, NDF-RT )
|
| presence of Problem Sick sinus syndrome (disorder) [SNOMED CT] | Absolute_contraindication | Calcium Channel blocker Non-Dihydropyridine Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex, NDF-RT )
|
| presence of Problem Wolff-Parkinson-White pattern (disorder) [SNOMED CT] | Absolute_contraindication | Calcium Channel blocker Non-Dihydropyridine Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex )
|
| presence of Problem First degree atrioventricular block (disorder) [SNOMED CT] | Relative_contraindication | Calcium Channel blocker Non-Dihydropyridine Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex, NDF-RT )
|
| presence of Problem Congestive heart failure (disorder) [SNOMED CT] | Relative_contraindication | Calcium Channel blocker Non-Dihydropyridine Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex )
|
| presence of Problem Acute myocardial infarction (disorder) [SNOMED CT] | Absolute_contraindication | Calcium Channel Blocker-Dihydropyridine Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex )
|
| presence of Problem Congestive heart failure (disorder) [SNOMED CT] | Relative_contraindication | Calcium Channel Blocker-Dihydropyridine Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex )
|
| presence of Problem Clonidine allergy (disorder) [SNOMED CT] | Absolute_contraindication | Clonidine (product) [SNOMED CT] |
Supplemental_Material ( Micromedex, NDF-RT, Epocrates )
|
| presence of Problem Cerebrovascular disease (disorder) [SNOMED CT] | Relative_contraindication | Clonidine (product) [SNOMED CT] |
Supplemental_Material ( Micromedex )
|
| presence of Problem Hemolytic anemia (disorder) [SNOMED CT] | Relative_contraindication | Methyldopa (product) [SNOMED CT] |
Supplemental_Material ( Micromedex )
|
| presence of Problem Disorder of liver (disorder) [SNOMED CT] | Absolute_contraindication | Guanfacine (product) [SNOMED CT] |
Supplemental_Material ( Micromedex, NDF-RT )
|
| presence of Problem Renal impairment (disorder) [SNOMED CT] | Relative_contraindication | Guanfacine (product) [SNOMED CT] |
Supplemental_Material ( Micromedex )
|
| presence of Problem Methyldopa allergy (disorder) [SNOMED CT] | Absolute_contraindication | Methyldopa (product) [SNOMED CT] |
Supplemental_Material ( Micromedex, NDF-RT, Epocrates )
|
| presence of Problem Ulcerative colitis (disorder) [SNOMED CT] | Absolute_contraindication | Reserpine (product) [SNOMED CT] |
Supplemental_Material ( Micromedex, NDF-RT, Epocrates )
|
| presence of Problem Thiazide diuretic allergy (disorder) [SNOMED CT] | Absolute_contraindication | Thiazide Diuretic Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex, NDF-RT, Epocrates )
|
| presence of Problem Allergy to sulfonamides (disorder) [SNOMED CT] | Relative_contraindication | Thiazide Diuretic Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex, NDF-RT, Epocrates )
|
| presence of Problem Anuria (finding) [SNOMED CT] | Absolute_contraindication | Thiazide Diuretic Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex, NDF-RT, Epocrates )
|
| presence of Observation Patient currently pregnant (finding) [SNOMED CT] | Relative_contraindication | Thiazide Diuretic Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( NDF-RT )
|
| presence of Problem Chlorthalidone allergy (disorder) [SNOMED CT] | Absolute_contraindication | Chlorthalidone (product) [SNOMED CT] |
Supplemental_Material ( Micromedex, NDF-RT, Epocrates )
|
| presence of Problem Gout (disorder) [SNOMED CT] | Relative_contraindication | Thiazide Diuretic Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex )
|
| presence of Problem Hypokalemia (disorder) [SNOMED CT] | Relative_contraindication | Thiazide Diuretic Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex )
|
| presence of Problem Hyponatremia (disorder) [SNOMED CT] | Relative_contraindication | Thiazide Diuretic Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex )
|
| presence of Problem Hypomagnesemia (disorder) [SNOMED CT] | Relative_contraindication | Thiazide Diuretic Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex )
|
| presence of Problem Hypercalcemia (disorder) [SNOMED CT] | Relative_contraindication | Thiazide Diuretic Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex )
|
| presence of Problem Hydrochlorothiazide allergy (disorder) [SNOMED CT] | Absolute_contraindication | Hydrochlorothiazide (product) [SNOMED CT] |
Supplemental_Material ( Micromedex, NDF-RT, Epocrates )
|
| presence of Problem Hyperuricemia (disorder) [SNOMED CT] | Relative_contraindication | Thiazide Diuretic Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex )
|
| presence of Problem Polythiazide allergy (disorder) [SNOMED CT] | Absolute_contraindication | Polythiazide (product) [SNOMED CT] |
Supplemental_Material ( Micromedex, NDF-RT, Epocrates )
|
| presence of Problem Indapamide allergy (disorder) [SNOMED CT] | Absolute_contraindication | Indapamide (product) [SNOMED CT] |
Supplemental_Material ( Micromedex, NDF-RT, Epocrates )
|
| presence of Problem Hepatic coma (disorder) [SNOMED CT] | Absolute_contraindication | Thiazide Diuretic Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex, NDF-RT, Epocrates )
|
| presence of Problem Loop diuretic allergy (disorder) [SNOMED CT] | Absolute_contraindication | Loop Diuretic Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex, NDF-RT, Epocrates )
|
| presence of Problem Bumetanide allergy (disorder) [SNOMED CT] | Absolute_contraindication | Bumetanide (product) [SNOMED CT] |
Supplemental_Material ( Micromedex, NDF-RT, Epocrates )
|
| presence of Problem Anuria (finding) [SNOMED CT] | Absolute_contraindication | Loop Diuretic Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex, NDF-RT, Epocrates )
|
| presence of Problem Hepatic coma (disorder) [SNOMED CT] | Absolute_contraindication | Loop Diuretic Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex )
|
| presence of Observation Patient currently pregnant (finding) [SNOMED CT] | Relative_contraindication | Loop Diuretic Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( NDF )
|
| presence of Problem Hyperuricemia (disorder) [SNOMED CT] | Relative_contraindication | Loop Diuretic Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex )
|
| presence of Problem Hypokalemia (disorder) [SNOMED CT] | Relative_contraindication | Loop Diuretic Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex )
|
| presence of Problem Hypomagnesemia (disorder) [SNOMED CT] | Relative_contraindication | Loop Diuretic Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex )
|
| presence of Problem Gout (disorder) [SNOMED CT] | Relative_contraindication | Loop Diuretic Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex )
|
| presence of Problem Triamterene allergy (disorder) [SNOMED CT] | Absolute_contraindication | Triamterene (product) [SNOMED CT] |
Supplemental_Material ( Micromedex, NDF-RT, Epocrates )
|
| presence of Problem Hyperkalemia (disorder) [SNOMED CT] | Absolute_contraindication | Potassium-sparing Diuretic Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex )
|
| presence of Problem Acidosis (disorder) [SNOMED CT] | Relative_contraindication | Potassium-sparing Diuretic Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex )
|
| presence of Problem Renal impairment (disorder) [SNOMED CT] | Absolute_contraindication | Potassium-sparing Diuretic Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex )
|
| presence of Problem Anuria (finding) [SNOMED CT] | Absolute_contraindication | Potassium-sparing Diuretic Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex, NDF-RT, Epocrates )
|
| presence of Observation Patient currently pregnant (finding) [SNOMED CT] | Relative_contraindication | Potassium-sparing Diuretic Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( NDF-RT )
|
| presence of Problem Disorder of liver (disorder) [SNOMED CT] | Relative_contraindication | Potassium-sparing Diuretic Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex, NDF-RT )
|
| presence of Problem Dissection of aorta (disorder) [SNOMED CT] | Absolute_contraindication | Hydralazine (product) [SNOMED CT] |
Supplemental_Material ( Micromedex )
|
| presence of Problem Minoxidil allergy (disorder) [SNOMED CT] | Absolute_contraindication | Minoxidil (product) [SNOMED CT] |
Supplemental_Material ( Micromedex, NDF-RT, Epocrates )
|
| presence of Problem Mitral valve disorder (disorder) [SNOMED CT] | Relative_contraindication | Hydralazine (product) [SNOMED CT] |
Supplemental_Material ( Micromedex, Epocrates )
|
| presence of Problem Coronary arteriosclerosis (disorder) [SNOMED CT] | Relative_contraindication | Direct Vasodilator Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex, Epocrates )
|
| presence of Problem Pheochromocytoma (disorder) [SNOMED CT] | Absolute_contraindication | Minoxidil (product) [SNOMED CT] |
Supplemental_Material ( Micromedex, NDF-RT, Epocrates )
|
| presence of Problem Pericardial effusion (disorder) [SNOMED CT] | Relative_contraindication | Direct Vasodilator Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex, Epocrates )
|
| presence of Problem Metolazone allergy (disorder) [SNOMED CT] | Absolute_contraindication | Metolazone (product) [SNOMED CT] |
Supplemental_Material ( Micromedex, NDF-RT, Epocrates )
|
| presence of Problem Hydralazine allergy (disorder) [SNOMED CT] | Absolute_contraindication | Hydralazine (product) [SNOMED CT] |
Supplemental_Material ( Micromedex, NDF-RT, Epocrates )
|
| presence of Problem Systemic lupus erythematosus (disorder) [SNOMED CT] | Absolute_contraindication | Hydralazine (product) [SNOMED CT] |
Supplemental_Material ( Micromedex )
|
| presence of Problem Acute myocardial infarction (disorder) [SNOMED CT] | Relative_contraindication | Direct Vasodilator Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex, NDF-RT )
|
| presence of Problem Angina pectoris (disorder) [SNOMED CT] | Relative_contraindication | Direct Vasodilator Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex )
|
| presence of Problem Renal failure syndrome (disorder) [SNOMED CT] | Relative_contraindication | Direct Vasodilator Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex )
|
| presence of Problem Hypertrichosis (disorder) [SNOMED CT] | Relative_contraindication | Minoxidil (product) [SNOMED CT] |
Supplemental_Material ( Micromedex )
|
| presence of Problem Orthostatic hypotension (disorder) [SNOMED CT] | Relative_contraindication | Alpha 1 Blocker Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex )
|
| presence of Problem H/O: angiotensin II receptor antagonist allergy (context-dependent category) [SNOMED CT] | Absolute_contraindication | Angiotensin II Antagonist Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex, NDF-RT, Epocrates )
|
| presence of Problem Hepatic failure (disorder) [SNOMED CT] | Relative_contraindication | Angiotensin II Antagonist Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex )
|
| presence of Problem Myasthenia gravis (disorder) [SNOMED CT] | Relative_contraindication | Nonselective Beta-Blocker Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex )
|
| presence of Problem Peripheral vascular disease (disorder) [SNOMED CT] | Relative_contraindication | Nonselective Beta-Blocker Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex )
|
| presence of Observation Patient currently pregnant (finding) [SNOMED CT] | Relative_contraindication | Nonselective Beta-Blocker Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( NDF-RT )
|
| presence of Problem Timolol allergy (disorder) [SNOMED CT] | Absolute_contraindication | Timolol (product) [SNOMED CT] |
Supplemental_Material ( Micromedex, NDF-RT, Epocrates )
|
| presence of Problem Propranolol allergy (disorder) [SNOMED CT] | Absolute_contraindication | Propranolol (product) [SNOMED CT] |
Supplemental_Material ( Micromedex, NDF-RT, Epocrates )
|
| presence of Observation Patient currently pregnant (finding) [SNOMED CT] | Relative_contraindication | Beta-blockers with Intrinsic Sympathomimetic Activity Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( NDF-RT )
|
| presence of Problem Sick sinus syndrome (disorder) [SNOMED CT] | Relative_contraindication | Beta-blockers with Intrinsic Sympathomimetic Activity Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( NDF-RT )
|
| presence of Problem Pulmonary edema [SAGE SNOMED CT] | Relative_contraindication | Beta-blockers with Intrinsic Sympathomimetic Activity Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( NDF-RT )
|
| presence of Problem Diabetes mellitus (disorder) [SNOMED CT] | Relative_contraindication | Beta-blockers with Intrinsic Sympathomimetic Activity Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex )
|
| presence of Problem Peripheral vascular disease (disorder) [SNOMED CT] | Relative_contraindication | Beta-blockers with Intrinsic Sympathomimetic Activity Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex )
|
| presence of Problem Penbutolol allergy (disorder) [SNOMED CT] | Absolute_contraindication | Penbutolol (product) [SNOMED CT] |
Supplemental_Material ( Micromedex, NDF-RT, Epocrates )
|
| presence of Problem Pindolol allergy (disorder) [SNOMED CT] | Absolute_contraindication | Pindolol (product) [SNOMED CT] |
Supplemental_Material ( Micromedex, NDF-RT, Epocrates )
|
| presence of Problem Atenolol allergy (disorder) [SNOMED CT] | Absolute_contraindication | Atenolol (product) [SNOMED CT] |
Supplemental_Material ( Micromedex, NDF-RT, Epocrates )
|
| presence of Problem Cardioselective beta-blocker allergy (disorder) [SNOMED CT] | Absolute_contraindication | Cardioselective Beta-Blocker Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex, NDF-RT, Epocrates )
|
| presence of Problem Bisoprolol allergy (disorder) [SNOMED CT] | Absolute_contraindication | Bisoprolol (product) [SNOMED CT] |
Supplemental_Material ( Micromedex, NDF-RT, Epocrates )
|
| presence of Problem Metoprolol allergy (disorder) [SNOMED CT] | Absolute_contraindication | Metoprolol (product) [SNOMED CT] |
Supplemental_Material ( Micromedex, NDF-RT, Epocrates )
|
| presence of Problem Nadolol allergy (disorder) [SNOMED CT] | Absolute_contraindication | Nadolol (product) [SNOMED CT] |
Supplemental_Material ( Micromedex, NDF-RT, Epocrates )
|
| presence of Problem Asthma (disorder) [SNOMED CT] | Relative_contraindication | Cardioselective Beta-Blocker Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex )
|
| presence of Observation Patient currently pregnant (finding) [SNOMED CT] | Relative_contraindication | Cardioselective Beta-Blocker Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex, NDF-RT )
|
| presence of Problem Diabetes mellitus (disorder) [SNOMED CT] | Relative_contraindication | Cardioselective Beta-Blocker Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex )
|
| presence of Problem Peripheral vascular disease (disorder) [SNOMED CT] | Relative_contraindication | Cardioselective Beta-Blocker Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex )
|
| presence of Problem Carvedilol allergy (disorder) [SNOMED CT] | Absolute_contraindication | Carvedilol (product) [SNOMED CT] |
Supplemental_Material ( Micromedex, NDF-RT, Epocrates )
|
| presence of Problem Orthostatic hypotension (disorder) [SNOMED CT] | Relative_contraindication | Combined Alpha- and Beta-Blocker Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( NDF-RT )
|
| presence of Problem Peripheral vascular disease (disorder) [SNOMED CT] | Relative_contraindication | Combined Alpha- and Beta-Blocker Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex )
|
| presence of Observation Patient currently pregnant (finding) [SNOMED CT] | Relative_contraindication | Combined Alpha- and Beta-Blocker Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( NDF-RT )
|
| presence of Problem Labetalol allergy (disorder) [SNOMED CT] | Absolute_contraindication | Labetalol (product) [SNOMED CT] |
Supplemental_Material ( Micromedex, NDF-RT, Epocrates )
|
| presence of Problem Wide QRS ventricular tachycardia (disorder) [SNOMED CT] | Absolute_contraindication | Calcium Channel blocker Non-Dihydropyridine Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex )
|
| presence of Problem Incomplete atrioventricular block (disorder) [SNOMED CT] | Absolute_contraindication | Calcium Channel blocker Non-Dihydropyridine Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex )
|
| presence of Problem Complete atrioventricular block (disorder) [SNOMED CT] | Absolute_contraindication | Calcium Channel blocker Non-Dihydropyridine Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex )
|
| presence of Problem Verapamil allergy (disorder) [SNOMED CT] | Absolute_contraindication | Verapamil (product) [SNOMED CT] |
Supplemental_Material ( Micromedex, NDF-RT, Epocrates )
|
| presence of Problem Amlodipine allergy (disorder) [SNOMED CT] | Absolute_contraindication | Amlodipine (product) [SNOMED CT] |
Supplemental_Material ( Micromedex, NDF-RT, Epocrates )
|
| presence of Problem Preinfarction syndrome (disorder) [SNOMED CT] | Absolute_contraindication | Calcium Channel Blocker-Dihydropyridine Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex )
|
| presence of Problem Aortic valve stenosis (disorder) [SNOMED CT] | Relative_contraindication | Calcium Channel Blocker-Dihydropyridine Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex )
|
| presence of Problem Peripheral edema (disorder) [SNOMED CT] | Relative_contraindication | Calcium Channel Blocker-Dihydropyridine Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex )
|
| presence of Problem Felodipine allergy (disorder) [SNOMED CT] | Absolute_contraindication | Felodipine (product) [SNOMED CT] |
Supplemental_Material ( Micromedex, NDF-RT, Epocrates )
|
| presence of Problem Isradipine allergy (disorder) [SNOMED CT] | Absolute_contraindication | Isradipine (product) [SNOMED CT] |
Supplemental_Material ( Micromedex, NDF-RT, Epocrates )
|
| presence of Problem Nicardipine allergy (disorder) [SNOMED CT] | Absolute_contraindication | Nicardipine (product) [SNOMED CT] |
Supplemental_Material ( Micromedex, NDF-RT, Epocrates )
|
| presence of Problem Nifedipine allergy (disorder) [SNOMED CT] | Absolute_contraindication | Nifedipine (product) [SNOMED CT] |
Supplemental_Material ( Micromedex, NDF-RT, Epocrates )
|
| presence of Problem Peptic ulcer (disorder) [SNOMED CT] | Absolute_contraindication | Reserpine (product) [SNOMED CT] |
Supplemental_Material ( NDF-RT )
|
| presence of Problem Depressive disorder (disorder) [SNOMED CT] | Absolute_contraindication | Reserpine (product) [SNOMED CT] |
Supplemental_Material ( Micromedex, NDF-RT, Epocrates )
|
| presence of Problem Rauwolfia antihypertensive allergy (disorder) [SNOMED CT] | Absolute_contraindication | Reserpine (product) [SNOMED CT] |
Supplemental_Material ( Micromedex, NDF-RT, Epocrates )
|
| presence of Problem Amiloride allergy (disorder) [SNOMED CT] | Absolute_contraindication | Amiloride (product) [SNOMED CT] |
Supplemental_Material ( Micromedex, NDF-RT, Epocrates )
|
| presence of Problem Hyperglycemia (disorder) [SNOMED CT] | Relative_contraindication | Loop Diuretic Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex )
|
| presence of Problem Frusemide allergy (disorder) [SNOMED CT] | Absolute_contraindication | Furosemide (product) [SNOMED CT] |
Supplemental_Material ( Micromedex, NDF-RT, Epocrates )
|
| presence of Problem Torasemide allergy (disorder) [SNOMED CT] | Absolute_contraindication | Torasemide (product) [SNOMED CT] |
Supplemental_Material ( Micromedex, NDF-RT, Epocrates )
|
| presence of Problem Hypertrophic cardiomyopathy (disorder) [SNOMED CT] | Relative_contraindication | Angiotensin II Antagonist Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex )
|
| presence of Problem Congestive heart failure (disorder) [SNOMED CT] | Indications | ACE Inhibitor Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex )
|
| presence of Problem Acute myocardial infarction (disorder) [SNOMED CT] | Indications | ACE Inhibitor Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex, Epocrates )
|
| presence of Problem Hypertensive disorder, systemic arterial (disorder) [SNOMED CT] | Indications | ACE Inhibitor Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex )
|
| presence of Problem Diabetic renal disease (disorder) [SNOMED CT] | Indications | ACE Inhibitor Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex )
|
| presence of Problem Congestive heart failure (disorder) [SNOMED CT] | Indications | Aldosterone Receptor Blocker Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex, Epocrates )
|
| presence of Problem Primary hyperaldosteronism (disorder) [SNOMED CT] | Indications | Aldosterone Receptor Blocker Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex, NDF-RT, Epocrates )
|
| presence of Problem Hypertensive disorder, systemic arterial (disorder) [SNOMED CT] | Indications | Aldosterone Receptor Blocker Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex, NDF-RT, Epocrates )
|
| presence of Problem Hepatic ascites (disorder) [SNOMED CT] | Indications | Aldosterone Receptor Blocker Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex )
|
| presence of Problem Hirsutism (disorder) [SNOMED CT] | Indications | Aldosterone Receptor Blocker Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex, NDF-RT )
|
| presence of Problem Hyperplasia of prostate (disorder) [SNOMED CT] | Indications | Alpha 1 Blocker Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex, NDF-RT, Epocrates )
|
| presence of Problem Congestive heart failure (disorder) [SNOMED CT] | Indications | Alpha 1 Blocker Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex )
|
| presence of Problem Open-angle glaucoma (disorder) [SNOMED CT] | Indications | Nonselective Beta-Blocker Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex, NDF-RT )
|
| presence of Problem Acute myocardial infarction (disorder) [SNOMED CT] | Indications | Nonselective Beta-Blocker Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex, Epocrates )
|
| presence of Problem Hypertensive disorder, systemic arterial (disorder) [SNOMED CT] | Indications | Nonselective Beta-Blocker Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex, NDF-RT, Epocrates )
|
| presence of Problem Angina pectoris (disorder) [SNOMED CT] | Indications | Nonselective Beta-Blocker Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex, NDF-RT, Epocrates )
|
| presence of Problem Ocular hypertension (disorder) [SNOMED CT] | Indications | Nonselective Beta-Blocker Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex, NDF-RT )
|
| presence of Problem Pheochromocytoma (disorder) [SNOMED CT] | Indications | Nonselective Beta-Blocker Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex, NDF-RT )
|
| presence of Problem Tachyarrhythmia (disorder) [SNOMED CT] | Indications | Nonselective Beta-Blocker Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex, NDF-RT, Epocrates )
|
| presence of Problem Migraine (disorder) [SNOMED CT] | Indications | Nonselective Beta-Blocker Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex, NDF-RT, Epocrates )
|
| presence of Problem Acute myocardial infarction (disorder) [SNOMED CT] | Indications | Beta-blockers with Intrinsic Sympathomimetic Activity Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex, Epocrates )
|
| presence of Problem Hypertensive disorder, systemic arterial (disorder) [SNOMED CT] | Indications | Beta-blockers with Intrinsic Sympathomimetic Activity Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex, NDF-RT, Epocrates )
|
| presence of Problem Angina pectoris (disorder) [SNOMED CT] | Indications | Beta-blockers with Intrinsic Sympathomimetic Activity Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex, NDF-RT )
|
| presence of Problem Tachyarrhythmia (disorder) [SNOMED CT] | Indications | Beta-blockers with Intrinsic Sympathomimetic Activity Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex, Epocrates )
|
| presence of Problem Acute myocardial infarction (disorder) [SNOMED CT] | Indications | Cardioselective Beta-Blocker Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex, Epocrates )
|
| presence of Problem Open-angle glaucoma (disorder) [SNOMED CT] | Indications | Cardioselective Beta-Blocker Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex, NDF-RT )
|
| presence of Problem Ocular hypertension (disorder) [SNOMED CT] | Indications | Cardioselective Beta-Blocker Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex, NDF-RT )
|
| presence of Problem Congestive heart failure (disorder) [SNOMED CT] | Indications | Cardioselective Beta-Blocker Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex )
|
| presence of Problem Hypertensive disorder, systemic arterial (disorder) [SNOMED CT] | Indications | Cardioselective Beta-Blocker Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex, NDF-RT, Epocrates )
|
| presence of Problem Angina pectoris (disorder) [SNOMED CT] | Indications | Cardioselective Beta-Blocker Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex, NDF-RT, Epocrates )
|
| presence of Problem Pheochromocytoma (disorder) [SNOMED CT] | Indications | Cardioselective Beta-Blocker Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex )
|
| presence of Problem Tachyarrhythmia (disorder) [SNOMED CT] | Indications | Cardioselective Beta-Blocker Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex, NDF-RT, Epocrates )
|
| presence of Problem Migraine (disorder) [SNOMED CT] | Indications | Cardioselective Beta-Blocker Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex )
|
| presence of Problem Congestive heart failure (disorder) [SNOMED CT] | Indications | Combined Alpha- and Beta-Blocker Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex, NDF-RT, Epocrates )
|
| presence of Problem Acute myocardial infarction (disorder) [SNOMED CT] | Indications | Combined Alpha- and Beta-Blocker Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex, Epocrates )
|
| presence of Problem Hypertensive disorder, systemic arterial (disorder) [SNOMED CT] | Indications | Combined Alpha- and Beta-Blocker Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex, NDF-RT, Epocrates )
|
| presence of Problem Angina pectoris (disorder) [SNOMED CT] | Indications | Combined Alpha- and Beta-Blocker Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex, NDF-RT )
|
| presence of Problem Pheochromocytoma (disorder) [SNOMED CT] | Indications | Combined Alpha- and Beta-Blocker Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex )
|
| presence of Problem Tachyarrhythmia (disorder) [SNOMED CT] | Indications | Combined Alpha- and Beta-Blocker Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex )
|
| presence of Problem Migraine (disorder) [SNOMED CT] | Indications | Combined Alpha- and Beta-Blocker Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex )
|
| presence of Problem Angina pectoris (disorder) [SNOMED CT] | Indications | Calcium Channel blocker Non-Dihydropyridine Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex, NDF-RT )
|
| presence of Problem Atrial flutter (disorder) [SNOMED CT] | Indications | Calcium Channel blocker Non-Dihydropyridine Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex )
|
| presence of Problem Hypertensive disorder, systemic arterial (disorder) [SNOMED CT] | Indications | Calcium Channel blocker Non-Dihydropyridine Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex, NDF-RT )
|
| presence of Problem Atrial fibrillation (disorder) [SNOMED CT] | Indications | Calcium Channel blocker Non-Dihydropyridine Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex )
|
| presence of Problem Angina pectoris (disorder) [SNOMED CT] | Indications | Calcium Channel Blocker-Dihydropyridine Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex, NDF-RT, Epocrates )
|
| presence of Problem Atrial flutter (disorder) [SNOMED CT] | Indications | Calcium Channel Blocker-Dihydropyridine Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex )
|
| presence of Problem Hypertensive disorder, systemic arterial (disorder) [SNOMED CT] | Indications | Calcium Channel Blocker-Dihydropyridine Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex, NDF-RT, Epocrates )
|
| presence of Problem Raynaud's syndrome (disorder) [SNOMED CT] | Indications | Calcium Channel Blocker-Dihydropyridine Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex )
|
| presence of Problem Raynaud's phenomenon (disorder) [SNOMED CT] | Indications | Calcium Channel Blocker-Dihydropyridine Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex )
|
| presence of Problem Raynaud's disease (disorder) [SNOMED CT] | Indications | Calcium Channel Blocker-Dihydropyridine Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex )
|
| presence of Problem Hypertensive disorder, systemic arterial (disorder) [SNOMED CT] | Indications | Clonidine (product) [SNOMED CT] |
Supplemental_Material ( Micromedex, NDF-RT, Epocrates )
|
| presence of Problem Gilles de la Tourette's syndrome (disorder) [SNOMED CT] | Indications | Clonidine (product) [SNOMED CT] |
Supplemental_Material ( Micromedex )
|
| presence of Problem Hollow visceral neuropathy (disorder) [SNOMED CT] | Indications | Clonidine (product) [SNOMED CT] |
Supplemental_Material ( Micromedex )
|
| presence of Problem Psychoactive substance-induced organic withdrawal (disorder) [SNOMED CT] | Indications | Clonidine (product) [SNOMED CT] |
Supplemental_Material ( NDF-RT )
|
| presence of Problem Hypertensive disorder, systemic arterial (disorder) [SNOMED CT] | Indications | Guanfacine (product) [SNOMED CT] |
Supplemental_Material ( Micromedex, NDF-RT, Epocrates )
|
| presence of Problem Hypertensive disorder, systemic arterial (disorder) [SNOMED CT] | Indications | Methyldopa (product) [SNOMED CT] |
Supplemental_Material ( Micromedex, NDF-RT, Epocrates )
|
| presence of Problem Hypertensive disorder, systemic arterial (disorder) [SNOMED CT] | Indications | Reserpine (product) [SNOMED CT] |
Supplemental_Material ( Micromedex, NDF-RT, Epocrates )
|
| presence of Observation Edema (finding) [SNOMED CT] | Indications | Thiazide Diuretic Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( NDF-RT )
|
| presence of Problem Hypertensive disorder, systemic arterial (disorder) [SNOMED CT] | Indications | Thiazide Diuretic Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex, NDF-RT, Epocrates )
|
| presence of Problem Hypercalciuria (disorder) [SNOMED CT] | Indications | Thiazide Diuretic Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex )
|
| presence of Problem Congestive heart failure (disorder) [SNOMED CT] | Indications | Thiazide Diuretic Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( NDF-RT )
|
| presence of Problem Hypertensive disorder, systemic arterial (disorder) [SNOMED CT] | Indications | Loop Diuretic Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex, NDF-RT, Epocrates )
|
| presence of Problem Congestive heart failure (disorder) [SNOMED CT] | Indications | Loop Diuretic Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex, NDF-RT )
|
| presence of Observation Edema (finding) [SNOMED CT] | Indications | Loop Diuretic Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex, NDF-RT, Epocrates )
|
| presence of Problem Nephrotic syndrome (disorder) [SNOMED CT] | Indications | Loop Diuretic Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex, NDF-RT )
|
| presence of Problem Renal impairment (disorder) [SNOMED CT] | Indications | Loop Diuretic Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Epocrates )
|
| presence of Problem Congestive heart failure (disorder) [SNOMED CT] | Indications | Potassium-sparing Diuretic Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex, Epocrates )
|
| presence of Problem Hypertensive disorder, systemic arterial (disorder) [SNOMED CT] | Indications | Potassium-sparing Diuretic Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex, NDF-RT, Epocrates )
|
| presence of Problem Hypokalemia (disorder) [SNOMED CT] | Indications | Potassium-sparing Diuretic Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( NDF-RT )
|
| presence of Problem Cystic fibrosis (disorder) [SNOMED CT] | Indications | Amiloride (product) [SNOMED CT] |
Supplemental_Material ( Micromedex )
|
| presence of Problem Atrial tachycardia (disorder) [SNOMED CT] | May_have_favorable_effects | Beta-Blocker Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( JNC 7 )
|
| presence of Problem Supraventricular tachycardia (disorder) [SNOMED CT] | May_have_favorable_effects | Calcium Channel blocker Non-Dihydropyridine Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( JNC 7 )
|
| presence of Problem Atrial fibrillation (disorder) [SNOMED CT] | May_have_favorable_effects | Beta-Blocker Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( JNC 7 )
|
| presence of Problem Atrial fibrillation (disorder) [SNOMED CT] | May_have_favorable_effects | Calcium Channel blocker Non-Dihydropyridine Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( JNC 7 )
|
| presence of Problem Essential tremor (finding) [SNOMED CT] | May_have_favorable_effects | Nonselective Beta-Blocker Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( JNC 7 )
|
| presence of Problem Hyperthyroidism (disorder) [SNOMED CT] | May_have_favorable_effects | Beta-Blocker Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( JNC 7 )
|
| presence of Problem Migraine (disorder) [SNOMED CT] | May_have_favorable_effects | Nonselective Beta-Blocker Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( JNC 7 )
|
| presence of Problem Osteoporosis (disorder) [SNOMED CT] | May_have_favorable_effects | Thiazide Diuretic Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( JNC 7 )
|
| presence of Problem Prostatism (disorder) [SNOMED CT] | May_have_favorable_effects | Alpha 1 Blocker Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( JNC 7 )
|
| presence of Problem Benign prostatic hyperplasia (disorder) [SNOMED CT] | May_have_favorable_effects | Alpha 1 Blocker Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( JNC 7 )
|
| presence of Problem Raynaud's syndrome (disorder) [SNOMED CT] | May_have_favorable_effects | Calcium Channel Blocker Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( JNC 7 )
|
| presence of Problem Type 1 diabetes mellitus with persistent microalbuminuria (disorder) [SNOMED CT] | May_have_favorable_effects | ACE Inhibitor Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( JNC 7 )
|
| presence of Problem Type 2 diabetes mellitus with persistent microalbuminuria (disorder) [SNOMED CT] | May_have_favorable_effects | ACE Inhibitor Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( JNC 7 )
|
| presence of Problem Type 2 diabetes mellitus with persistent microalbuminuria (disorder) [SNOMED CT] | May_have_favorable_effects | Angiotensin II Antagonist Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( JNC 7 )
|
| presence of Problem Postmenopausal flushing (disorder) [SNOMED CT] | Indications | Clonidine (product) [SNOMED CT] |
Supplemental_Material ( Micromedex )
|
| presence of Problem Postmenopausal flushing (disorder) [SNOMED CT] | Indications | Methyldopa (product) [SNOMED CT] |
Supplemental_Material ( Micromedex )
|
| presence of Problem Osteoporosis (disorder) [SNOMED CT] | Indications | Thiazide Diuretic Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex )
|
| presence of Problem Osteopenia (disorder) [SNOMED CT] | Indications | Thiazide Diuretic Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex )
|
| presence of Problem Congestive heart failure (disorder) [SNOMED CT] | Indications | Triamterene (product) [SNOMED CT] |
Supplemental_Material ( Micromedex )
|
| presence of Problem Hypertensive disorder, systemic arterial (disorder) [SNOMED CT] | Indications | Triamterene (product) [SNOMED CT] |
Supplemental_Material ( Micromedex, NDF-RT )
|
| presence of Problem Pulmonary edema [SAGE SNOMED CT] | Indications | Loop Diuretic Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( NDF-RT )
|
| presence of Problem Betaxolol allergy (disorder) [SNOMED CT] | Absolute_contraindication | Betaxolol (product) [SNOMED CT] |
Supplemental_Material ( Micromedex, NDF-RT, Epocrates )
|
| presence of Problem Acute myocardial infarction (disorder) [SNOMED CT] | Absolute_contraindication | Clonidine (product) [SNOMED CT] |
Supplemental_Material ( Micromedex )
|
| presence of Problem Acute myocardial infarction (disorder) [SNOMED CT] | Relative_contraindication | Guanfacine (product) [SNOMED CT] |
Supplemental_Material ( Micromedex )
|
| presence of Problem Cerebrovascular disease (disorder) [SNOMED CT] | Relative_contraindication | Guanfacine (product) [SNOMED CT] |
Supplemental_Material ( Micromedex )
|
| presence of Problem Disorder of liver (disorder) [SNOMED CT] | Absolute_contraindication | Methyldopa (product) [SNOMED CT] |
Supplemental_Material ( Micromedex, NDF-RT )
|
| presence of Problem Disorder of liver (disorder) [SNOMED CT] | Absolute_contraindication | Reserpine (product) [SNOMED CT] |
Supplemental_Material ( Micromedex )
|
| presence of Problem ACE inhibitor-aggravated angioedema (disorder) [SNOMED CT] | Absolute_contraindication | ACE Inhibitor Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex )
|
| presence of Problem Anuria (finding) [SNOMED CT] | Absolute_contraindication | ACE Inhibitor Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex, NDF-RT, Epocrates )
|
| presence of Problem Heart failure (disorder) [SNOMED CT] | Relative_contraindication | Nonselective Beta-Blocker Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex )
|
| presence of Problem Asthma (disorder) [SNOMED CT] | Absolute_contraindication | Nonselective Beta-Blocker Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex )
|
| presence of Problem Calcium-channel blocker allergy (disorder) [SNOMED CT] | Absolute_contraindication | Nisoldipine (product) [SNOMED CT] |
Supplemental_Material ( Micromedex, NDF-RT, Epocrates )
|
| presence of Problem Sick sinus syndrome (disorder) [SNOMED CT] | Absolute_contraindication | Nonselective Beta-Blocker Oral Preparation for Hypertension [SAGE NDF-RT] |
Supplemental_Material ( Micromedex )
|
| presence of Problem Tetracyclines group allergy (disorder) [SNOMED CT] | Absolute_contraindication | Doxycycline Preparation for CAP [SAGE NDF-RT] |
Supplemental_Material ( Micromedex )
|
| presence of Problem Doxycycline allergy (disorder) [SNOMED CT] | Absolute_contraindication | Doxycycline Preparation for CAP [SAGE NDF-RT] |
Supplemental_Material ( Micromedex, NDF-RT, Epocrates )
|
| presence of Observation Patient currently pregnant (finding) [SNOMED CT] | Absolute_contraindication | Doxycycline Preparation for CAP [SAGE NDF-RT] |
Supplemental_Material ( Micromedex, NDF-RT )
|
| presence of Problem Hepatic failure (disorder) [SNOMED CT] | Relative_contraindication | Doxycycline Preparation for CAP [SAGE NDF-RT] |
Supplemental_Material ( Micromedex, NDF-RT, Epocrates )
|
| presence of Problem Clindamycin allergy (disorder) [SNOMED CT] | Absolute_contraindication | Clindamycin Preparation for CAP [SAGE NDF-RT] |
Supplemental_Material ( Micromedex, NDF-RT, Epocrates )
|
| presence of Problem Lincomycin allergy (disorder) [SNOMED CT] | Absolute_contraindication | Clindamycin Preparation for CAP [SAGE NDF-RT] |
Supplemental_Material ( Micromedex )
|
| presence of Problem Antibiotic enterocolitis (disorder) [SNOMED CT] | Absolute_contraindication | Clindamycin Preparation for CAP [SAGE NDF-RT] |
Supplemental_Material ( Micromedex, Epocrates )
|
| presence of Problem Pseudomembranous enterocolitis (disorder) [SNOMED CT] | Relative_contraindication | Clindamycin Preparation for CAP [SAGE NDF-RT] |
Supplemental_Material ( NDF-RT )
|
| presence of Problem Atopy (disorder) [SNOMED CT] | Relative_contraindication | Clindamycin Oral Preparation [SAGE NDF-RT] |
Supplemental_Material ( Micromedex )
|
| presence of Problem Renal impairment (disorder) [SNOMED CT] | Relative_contraindication | Clindamycin Preparation for CAP [SAGE NDF-RT] |
Supplemental_Material ( Epocrates )
|
| presence of Problem Hepatic failure (disorder) [SNOMED CT] | Relative_contraindication | Clindamycin Preparation for CAP [SAGE NDF-RT] |
Supplemental_Material ( Micromedex, NDF-RT, Epocrates )
|
| presence of Problem Crohn's disease (disorder) [SNOMED CT] | Absolute_contraindication | Clindamycin Parenteral Preparation [SAGE NDF-RT] |
Supplemental_Material ( Micromedex )
|
| presence of Problem Ulcerative colitis (disorder) [SNOMED CT] | Absolute_contraindication | Clindamycin Parenteral Preparation [SAGE NDF-RT] |
Supplemental_Material ( Micromedex )
|
| presence of Problem Allergy to erythromycin (disorder) [SNOMED CT] | Absolute_contraindication | Erythromycin Preparation for CAP [SAGE NDF-RT] |
Supplemental_Material ( Micromedex, NDF-RT, Epocrates )
|
| presence of Problem Hepatic failure (disorder) [SNOMED CT] | Relative_contraindication | Erythromycin Preparation for CAP [SAGE NDF-RT] |
Supplemental_Material ( Micromedex, NDF-RT, Epocrates )
|
| presence of Problem Myasthenia gravis (disorder) [SNOMED CT] | Relative_contraindication | Erythromycin Preparation for CAP [SAGE NDF-RT] |
Supplemental_Material ( Micromedex, Epocrates )
|
| presence of Problem Prolonged QT interval (finding) [SNOMED CT] | Relative_contraindication | Erythromycin Preparation for CAP [SAGE NDF-RT] |
Supplemental_Material ( Epocrates )
|
| presence of Problem Hypokalemia (disorder) [SNOMED CT] | Relative_contraindication | Erythromycin Preparation for CAP [SAGE NDF-RT] |
Supplemental_Material ( Epocrates )
|
| presence of Problem Hypomagnesemia (disorder) [SNOMED CT] | Relative_contraindication | Erythromycin Preparation for CAP [SAGE NDF-RT] |
Supplemental_Material ( Epocrates )
|
| presence of Problem Bradycardia (disorder) [SNOMED CT] | Relative_contraindication | Erythromycin Preparation for CAP [SAGE NDF-RT] |
Supplemental_Material ( Epocrates )
|
| presence of Problem Acute myocardial infarction (disorder) [SNOMED CT] | Relative_contraindication | Erythromycin Preparation for CAP [SAGE NDF-RT] |
Supplemental_Material ( Epocrates )
|
| presence of Problem Acute coronary syndrome (disorder) [SNOMED CT] | Relative_contraindication | Erythromycin Preparation for CAP [SAGE NDF-RT] |
Supplemental_Material ( Epocrates )
|
| presence of Problem Cardiomyopathy (disorder) [SNOMED CT] | Relative_contraindication | Erythromycin Preparation for CAP [SAGE NDF-RT] |
Supplemental_Material ( Epocrates )
|
| presence of Problem Azithromycin allergy (disorder) [SNOMED CT] | Absolute_contraindication | Azithromycin Preparation for CAP [SAGE NDF-RT] |
Supplemental_Material ( Micromedex, NDF-RT, Epocrates )
|
| presence of Problem Allergy to macrolide antibiotic (disorder) [SNOMED CT] | Absolute_contraindication | Azithromycin Preparation for CAP [SAGE NDF-RT] |
Supplemental_Material ( Micromedex, NDF-RT, Epocrates )
|
| presence of Problem Hepatic failure (disorder) [SNOMED CT] | Relative_contraindication | Azithromycin Preparation for CAP [SAGE NDF-RT] |
Supplemental_Material ( Micromedex, NDF-RT, Epocrates )
|
| presence of Problem End stage renal disease (disorder) [SNOMED CT] | Relative_contraindication | Azithromycin Preparation for CAP [SAGE NDF-RT] |
Supplemental_Material ( Micromedex, Epocrates )
|
| presence of Problem Prolonged QT interval (finding) [SNOMED CT] | Relative_contraindication | Azithromycin Preparation for CAP [SAGE NDF-RT] |
Supplemental_Material ( Micromedex )
|
| presence of Problem Clarithromycin allergy (disorder) [SNOMED CT] | Absolute_contraindication | Clarithromycin Preparation for CAP [SAGE NDF-RT] |
Supplemental_Material ( Micromedex, NDF-RT, Epocrates )
|
| presence of Problem Allergy to erythromycin (disorder) [SNOMED CT] | Absolute_contraindication | Clarithromycin Preparation for CAP [SAGE NDF-RT] |
Supplemental_Material ( Micromedex )
|
| presence of Problem Allergy to macrolide antibiotic (disorder) [SNOMED CT] | Absolute_contraindication | Clarithromycin Preparation for CAP [SAGE NDF-RT] |
Supplemental_Material ( Micromedex, NDF-RT, Epocrates )
|
| presence of Problem Acute intermittent porphyria (disorder) [SNOMED CT] | Relative_contraindication | Clarithromycin Preparation for CAP [SAGE NDF-RT] |
Supplemental_Material ( Micromedex, NDF-RT )
|
| presence of Observation Patient currently pregnant (finding) [SNOMED CT] | Relative_contraindication | Clarithromycin Preparation for CAP [SAGE NDF-RT] |
Supplemental_Material ( Micromedex )
|
| presence of Problem Renal impairment (disorder) [SNOMED CT] | Relative_contraindication | Clarithromycin Preparation for CAP [SAGE NDF-RT] |
Supplemental_Material ( Micromedex, Epocrates )
|
| presence of Problem Prolonged QT interval (finding) [SNOMED CT] | Relative_contraindication | Clarithromycin Preparation for CAP [SAGE NDF-RT] |
Supplemental_Material ( Epocrates )
|
| presence of Problem Hypokalemia (disorder) [SNOMED CT] | Relative_contraindication | Clarithromycin Preparation for CAP [SAGE NDF-RT] |
Supplemental_Material ( Epocrates )
|
| presence of Problem Hypomagnesemia (disorder) [SNOMED CT] | Relative_contraindication | Clarithromycin Preparation for CAP [SAGE NDF-RT] |
Supplemental_Material ( Epocrates )
|
| presence of Problem Bradycardia (disorder) [SNOMED CT] | Relative_contraindication | Clarithromycin Preparation for CAP [SAGE NDF-RT] |
Supplemental_Material ( Epocrates )
|
| presence of Problem Acute myocardial infarction (disorder) [SNOMED CT] | Relative_contraindication | Clarithromycin Preparation for CAP [SAGE NDF-RT] |
Supplemental_Material ( Epocrates )
|
| presence of Problem Acute coronary syndrome (disorder) [SNOMED CT] | Relative_contraindication | Clarithromycin Preparation for CAP [SAGE NDF-RT] |
Supplemental_Material ( Epocrates )
|
| presence of Problem 4-quinolones allergy (disorder) [SNOMED CT] | Absolute_contraindication | Moxifloxacin Preparation for CAP [SAGE NDF-RT] |
Supplemental_Material ( Micromedex, NDF-RT, Epocrates )
|
| presence of Observation Patient currently pregnant (finding) [SNOMED CT] | Absolute_contraindication | Moxifloxacin Preparation for CAP [SAGE NDF-RT] |
Supplemental_Material ( Epocrates )
|
| presence of Problem Prolonged QT interval (finding) [SNOMED CT] | Relative_contraindication | Moxifloxacin Preparation for CAP [SAGE NDF-RT] |
Supplemental_Material ( Micromedex, NDF-RT, Epocrates )
|
| presence of Problem Hypokalemia (disorder) [SNOMED CT] | Relative_contraindication | Moxifloxacin Preparation for CAP [SAGE NDF-RT] |
Supplemental_Material ( Micromedex, Epocrates )
|
| presence of Problem Epilepsy (disorder) [SNOMED CT] | Relative_contraindication | Moxifloxacin Preparation for CAP [SAGE NDF-RT] |
Supplemental_Material ( Micromedex, Epocrates )
|
| presence of Problem 4-quinolones allergy (disorder) [SNOMED CT] | Absolute_contraindication | Gatifloxacin Preparation for CAP [SAGE NDF-RT] |
Supplemental_Material ( Micromedex, NDF-RT, Epocrates )
|
| presence of Observation Patient currently pregnant (finding) [SNOMED CT] | Absolute_contraindication | Gatifloxacin Preparation for CAP [SAGE NDF-RT] |
Supplemental_Material ( Epocrates )
|
| presence of Problem Prolonged QT interval (finding) [SNOMED CT] | Relative_contraindication | Gatifloxacin Preparation for CAP [SAGE NDF-RT] |
Supplemental_Material ( Micromedex, Epocrates )
|
| presence of Problem Hypokalemia (disorder) [SNOMED CT] | Relative_contraindication | Gatifloxacin Preparation for CAP [SAGE NDF-RT] |
Supplemental_Material ( Micromedex, Epocrates )
|
| presence of Problem Acute myocardial infarction (disorder) [SNOMED CT] | Relative_contraindication | Gatifloxacin Preparation for CAP [SAGE NDF-RT] |
Supplemental_Material ( Micromedex )
|
| presence of Problem Acute coronary syndrome (disorder) [SNOMED CT] | Relative_contraindication | Gatifloxacin Preparation for CAP [SAGE NDF-RT] |
Supplemental_Material ( Micromedex )
|
| presence of Problem Cerebral arteriosclerosis (disorder) [SNOMED CT] | Relative_contraindication | Gatifloxacin Preparation for CAP [SAGE NDF-RT] |
Supplemental_Material ( Micromedex )
|
| presence of Problem Epilepsy (disorder) [SNOMED CT] | Relative_contraindication | Gatifloxacin Preparation for CAP [SAGE NDF-RT] |
Supplemental_Material ( Micromedex, Epocrates )
|
| presence of Problem Diabetes mellitus (disorder) [SNOMED CT] | Relative_contraindication | Gatifloxacin Preparation for CAP [SAGE NDF-RT] |
Supplemental_Material ( Micromedex, Epocrates )
|
| presence of Problem Renal failure syndrome (disorder) [SNOMED CT] | Relative_contraindication | Gatifloxacin Preparation for CAP [SAGE NDF-RT] |
Supplemental_Material ( Micromedex, Epocrates )
|
| presence of Problem End stage renal failure on dialysis (disorder) [SNOMED CT] | Relative_contraindication | Gatifloxacin Preparation for CAP [SAGE NDF-RT] |
Supplemental_Material ( Micromedex )
|
| presence of Problem Dyslipidemia (disorder) [SNOMED CT] | Relative_contraindication | Gatifloxacin Preparation for CAP [SAGE NDF-RT] |
Supplemental_Material ( Micromedex )
|
| presence of Problem Renal impairment (disorder) [SNOMED CT] | Relative_contraindication | Gatifloxacin Preparation for CAP [SAGE NDF-RT] |
Supplemental_Material ( Epocrates )
|
| presence of Problem 4-quinolones allergy (disorder) [SNOMED CT] | Absolute_contraindication | Levofloxacin Preparation for CAP [SAGE NDF-RT] |
Supplemental_Material ( Micromedex, NDF-RT, Epocrates )
|
| presence of Observation Patient currently pregnant (finding) [SNOMED CT] | Absolute_contraindication | Levofloxacin Preparation for CAP [SAGE NDF-RT] |
Supplemental_Material ( Epocrates )
|
| presence of Problem Renal failure syndrome (disorder) [SNOMED CT] | Relative_contraindication | Levofloxacin Preparation for CAP [SAGE NDF-RT] |
Supplemental_Material ( Micromedex )
|
| presence of Problem Prolonged QT interval (finding) [SNOMED CT] | Relative_contraindication | Levofloxacin Preparation for CAP [SAGE NDF-RT] |
Supplemental_Material ( Micromedex, Epocrates )
|
| presence of Problem Hypokalemia (disorder) [SNOMED CT] | Relative_contraindication | Levofloxacin Preparation for CAP [SAGE NDF-RT] |
Supplemental_Material ( Micromedex, Epocrates )
|
| presence of Problem End stage renal failure on dialysis (disorder) [SNOMED CT] | Relative_contraindication | Levofloxacin Preparation for CAP [SAGE NDF-RT] |
Supplemental_Material ( Micromedex )
|
| presence of Problem Dyslipidemia (disorder) [SNOMED CT] | Relative_contraindication | Levofloxacin Preparation for CAP [SAGE NDF-RT] |
Supplemental_Material ( Micromedex )
|
| presence of Problem Diabetes mellitus (disorder) [SNOMED CT] | Relative_contraindication | Levofloxacin Preparation for CAP [SAGE NDF-RT] |
Supplemental_Material ( Micromedex, Epocrates )
|
| presence of Problem Epilepsy (disorder) [SNOMED CT] | Relative_contraindication | Levofloxacin Preparation for CAP [SAGE NDF-RT] |
Supplemental_Material ( Micromedex, Epocrates )
|
| presence of Problem 4-quinolones allergy (disorder) [SNOMED CT] | Absolute_contraindication | Gemifloxacin Preparation for CAP [SAGE NDF-RT] |
Supplemental_Material ( Micromedex, NDF-RT, Epocrates )
|
| presence of Problem Prolonged QT interval (finding) [SNOMED CT] | Relative_contraindication | Gemifloxacin Preparation for CAP [SAGE NDF-RT] |
Supplemental_Material ( Micromedex, NDF-RT, Epocrates )
|
| presence of Problem Hypokalemia (disorder) [SNOMED CT] | Relative_contraindication | Gemifloxacin Preparation for CAP [SAGE NDF-RT] |
Supplemental_Material ( Micromedex, Epocrates )
|
| presence of Problem Hypomagnesemia (disorder) [SNOMED CT] | Relative_contraindication | Gemifloxacin Preparation for CAP [SAGE NDF-RT] |
Supplemental_Material ( Micromedex, Epocrates )
|
| presence of Problem Bradycardia (disorder) [SNOMED CT] | Relative_contraindication | Gemifloxacin Preparation for CAP [SAGE NDF-RT] |
Supplemental_Material ( Micromedex )
|
| presence of Problem Acute myocardial infarction (disorder) [SNOMED CT] | Relative_contraindication | Gemifloxacin Preparation for CAP [SAGE NDF-RT] |
Supplemental_Material ( Micromedex )
|
| presence of Problem Acute coronary syndrome (disorder) [SNOMED CT] | Relative_contraindication | Gemifloxacin Preparation for CAP [SAGE NDF-RT] |
Supplemental_Material ( Micromedex )
|
| presence of Problem Epilepsy (disorder) [SNOMED CT] | Relative_contraindication | Gemifloxacin Preparation for CAP [SAGE NDF-RT] |
Supplemental_Material ( Micromedex, Epocrates )
|
| presence of Problem Renal impairment (disorder) [SNOMED CT] | Relative_contraindication | Gemifloxacin Preparation for CAP [SAGE NDF-RT] |
Supplemental_Material ( Epocrates )
|
| presence of Observation Patient currently pregnant (finding) [SNOMED CT] | Relative_contraindication | Gemifloxacin Preparation for CAP [SAGE NDF-RT] |
Supplemental_Material ( Micromedex, NDF-RT, Epocrates )
|
| presence of Problem Allergy to penicillin (disorder) [SNOMED CT] | Absolute_contraindication | Amoxicillin Preparation for CAP [SAGE NDF-RT] |
Supplemental_Material ( Micromedex, NDF-RT, Epocrates )
|
| presence of Problem Cephalosporin allergy (disorder) [SNOMED CT] | Relative_contraindication | Amoxicillin Preparation for CAP [SAGE NDF-RT] |
Supplemental_Material ( Micromedex, NDF-RT, Epocrates )
|
| presence of Problem Classical phenylketonuria (disorder) [SNOMED CT] | Relative_contraindication | Amoxicillin Preparation for CAP [SAGE NDF-RT] |
Supplemental_Material ( Micromedex )
|
| presence of Problem Renal impairment (disorder) [SNOMED CT] | Relative_contraindication | Amoxicillin Preparation for CAP [SAGE NDF-RT] |
Supplemental_Material ( Epocrates )
|
| presence of Problem Hepatic failure (disorder) [SNOMED CT] | Relative_contraindication | Amoxicillin-Clavulanate Oral Preparation [SAGE NDF-RT] |
Supplemental_Material ( Micromedex, NDF-RT, Epocrates )
|
| presence of Problem Obstruction of bile duct (disorder) [SNOMED CT] | Relative_contraindication | Amoxicillin-Clavulanate Oral Preparation [SAGE NDF-RT] |
Supplemental_Material ( Epocrates )
|
| presence of Problem Cefpodoxime allergy (disorder) [SNOMED CT] | Absolute_contraindication | Cefpodoxime Preparation for CAP [SAGE NDF-RT] |
Supplemental_Material ( Micromedex, NDF-RT, Epocrates )
|
| presence of Problem Cephalosporin allergy (disorder) [SNOMED CT] | Absolute_contraindication | Cefpodoxime Preparation for CAP [SAGE NDF-RT] |
Supplemental_Material ( Micromedex, NDF-RT, Epocrates )
|
| presence of Problem Allergy to penicillin (disorder) [SNOMED CT] | Relative_contraindication | Cefpodoxime Preparation for CAP [SAGE NDF-RT] |
Supplemental_Material ( Micromedex )
|
| presence of Problem Pseudomembranous enterocolitis (disorder) [SNOMED CT] | Relative_contraindication | Cefpodoxime Preparation for CAP [SAGE NDF-RT] |
Supplemental_Material ( Micromedex, Epocrates )
|
| presence of Problem Renal impairment (disorder) [SNOMED CT] | Relative_contraindication | Cefpodoxime Preparation for CAP [SAGE NDF-RT] |
Supplemental_Material ( Epocrates )
|
| presence of Problem Epilepsy (disorder) [SNOMED CT] | Relative_contraindication | Cefpodoxime Preparation for CAP [SAGE NDF-RT] |
Supplemental_Material ( Epocrates )
|
| presence of Problem Cephalosporin allergy (disorder) [SNOMED CT] | Absolute_contraindication | Cefprozil Preparation for CAP [SAGE NDF-RT] |
Supplemental_Material ( Micromedex, NDF-RT, Epocrates )
|
| presence of Problem Allergy to penicillin (disorder) [SNOMED CT] | Relative_contraindication | Cefprozil Preparation for CAP [SAGE NDF-RT] |
Supplemental_Material ( Micromedex )
|
| presence of Problem Pseudomembranous enterocolitis (disorder) [SNOMED CT] | Relative_contraindication | Cefprozil Preparation for CAP [SAGE NDF-RT] |
Supplemental_Material ( Micromedex )
|
| presence of Problem Classical phenylketonuria (disorder) [SNOMED CT] | Relative_contraindication | Cefprozil Preparation for CAP [SAGE NDF-RT] |
Supplemental_Material ( Micromedex )
|
| presence of Problem Renal impairment (disorder) [SNOMED CT] | Relative_contraindication | Cefprozil Preparation for CAP [SAGE NDF-RT] |
Supplemental_Material ( Epocrates )
|
| presence of Problem Epilepsy (disorder) [SNOMED CT] | Relative_contraindication | Cefprozil Preparation for CAP [SAGE NDF-RT] |
Supplemental_Material ( Epocrates )
|
| presence of Problem Cephalosporin allergy (disorder) [SNOMED CT] | Absolute_contraindication | Cefuroxime Preparation for CAP [SAGE NDF-RT] |
Supplemental_Material ( Micromedex, NDF-RT, Epocrates )
|
| presence of Problem Cefuroxime allergy (disorder) [SNOMED CT] | Absolute_contraindication | Cefuroxime Preparation for CAP [SAGE NDF-RT] |
Supplemental_Material ( Micromedex, NDF-RT, Epocrates )
|
| presence of Problem Allergy to penicillin (disorder) [SNOMED CT] | Relative_contraindication | Cefuroxime Preparation for CAP [SAGE NDF-RT] |
Supplemental_Material ( Micromedex )
|
| presence of Problem Pseudomembranous enterocolitis (disorder) [SNOMED CT] | Relative_contraindication | Cefuroxime Preparation for CAP [SAGE NDF-RT] |
Supplemental_Material ( Micromedex, Epocrates )
|
| presence of Problem Renal impairment (disorder) [SNOMED CT] | Relative_contraindication | Cefuroxime Preparation for CAP [SAGE NDF-RT] |
Supplemental_Material ( Epocrates )
|
| presence of Problem Epilepsy (disorder) [SNOMED CT] | Relative_contraindication | Cefuroxime Preparation for CAP [SAGE NDF-RT] |
Supplemental_Material ( Epocrates )
|
| presence of Problem Ampicillin allergy (disorder) [SNOMED CT] | Absolute_contraindication | Ampicillin-Sulbactam Preparation for CAP [SAGE NDF-RT] |
Supplemental_Material ( Micromedex, NDF-RT, Epocrates )
|
| presence of Problem Allergy to penicillin (disorder) [SNOMED CT] | Absolute_contraindication | Ampicillin-Sulbactam Preparation for CAP [SAGE NDF-RT] |
Supplemental_Material ( Micromedex, NDF-RT, Epocrates )
|
| presence of Problem Cephalosporin allergy (disorder) [SNOMED CT] | Relative_contraindication | Ampicillin-Sulbactam Preparation for CAP [SAGE NDF-RT] |
Supplemental_Material ( Micromedex, Epocrates )
|
| presence of Problem Infectious mononucleosis (disorder) [SNOMED CT] | Relative_contraindication | Ampicillin-Sulbactam Preparation for CAP [SAGE NDF-RT] |
Supplemental_Material ( Micromedex )
|
| presence of Problem Renal impairment (disorder) [SNOMED CT] | Relative_contraindication | Ampicillin-Sulbactam Preparation for CAP [SAGE NDF-RT] |
Supplemental_Material ( Epocrates )
|
| presence of Problem Carbapenem allergy (disorder) [SNOMED CT] | Absolute_contraindication | Ertapenem Preparation for CAP [SAGE NDF-RT] |
Supplemental_Material ( Micromedex, NDF-RT, Epocrates )
|
| presence of Problem Lignocaine allergy (disorder) [SNOMED CT] | Absolute_contraindication | Ertapenem Preparation for CAP [SAGE NDF-RT] |
Supplemental_Material ( Micromedex, Epocrates )
|
| presence of Problem Epilepsy (disorder) [SNOMED CT] | Relative_contraindication | Ertapenem Preparation for CAP [SAGE NDF-RT] |
Supplemental_Material ( Micromedex, Epocrates )
|
| presence of Problem Renal impairment (disorder) [SNOMED CT] | Relative_contraindication | Ertapenem Preparation for CAP [SAGE NDF-RT] |
Supplemental_Material ( Epocrates )
|
| presence of Problem Cephalosporin allergy (disorder) [SNOMED CT] | Absolute_contraindication | Cefotaxime Preparation for CAP [SAGE NDF-RT] |
Supplemental_Material ( Micromedex, NDF-RT, Epocrates )
|
| presence of Problem Cefotaxime allergy (disorder) [SNOMED CT] | Absolute_contraindication | Cefotaxime Preparation for CAP [SAGE NDF-RT] |
Supplemental_Material ( Micromedex, NDF-RT, Epocrates )
|
| presence of Problem Allergy to penicillin (disorder) [SNOMED CT] | Relative_contraindication | Cefotaxime Preparation for CAP [SAGE NDF-RT] |
Supplemental_Material ( Micromedex, NDF-RT, Epocrates )
|
| presence of Problem Pseudomembranous enterocolitis (disorder) [SNOMED CT] | Relative_contraindication | Cefotaxime Preparation for CAP [SAGE NDF-RT] |
Supplemental_Material ( Micromedex )
|
| presence of Problem Renal impairment (disorder) [SNOMED CT] | Relative_contraindication | Cefotaxime Preparation for CAP [SAGE NDF-RT] |
Supplemental_Material ( Epocrates )
|
| presence of Problem Epilepsy (disorder) [SNOMED CT] | Relative_contraindication | Cefotaxime Preparation for CAP [SAGE NDF-RT] |
Supplemental_Material ( Epocrates )
|
| presence of Problem Cephalosporin allergy (disorder) [SNOMED CT] | Absolute_contraindication | Ceftriaxone Preparation for CAP [SAGE NDF-RT] |
Supplemental_Material ( Micromedex, NDF-RT, Epocrates )
|
| presence of Problem Ceftriaxone allergy (disorder) [SNOMED CT] | Absolute_contraindication | Ceftriaxone Preparation for CAP [SAGE NDF-RT] |
Supplemental_Material ( Micromedex, NDF-RT, Epocrates )
|
| presence of Problem Neonatal hyperbilirubinemia (disorder) [SNOMED CT] | Absolute_contraindication | Ceftriaxone Parenteral Preparation [SAGE NDF-RT] |
Supplemental_Material ( Micromedex, NDF-RT, Epocrates )
|
| presence of Problem Allergy to penicillin (disorder) [SNOMED CT] | Relative_contraindication | Ceftriaxone Preparation for CAP [SAGE NDF-RT] |
Supplemental_Material ( Micromedex )
|
| presence of Problem Pseudomembranous enterocolitis (disorder) [SNOMED CT] | Relative_contraindication | Ceftriaxone Preparation for CAP [SAGE NDF-RT] |
Supplemental_Material ( Micromedex )
|
| presence of Problem Disorder of gallbladder (disorder) [SNOMED CT] | Relative_contraindication | Ceftriaxone Preparation for CAP [SAGE NDF-RT] |
Supplemental_Material ( NDF-RT )
|
| presence of Problem Epilepsy (disorder) [SNOMED CT] | Relative_contraindication | Ceftriaxone Preparation for CAP [SAGE NDF-RT] |
Supplemental_Material ( Epocrates )
|
| presence of Problem Renal impairment (disorder) [SNOMED CT] | Relative_contraindication | Ceftriaxone Preparation for CAP [SAGE NDF-RT] |
Supplemental_Material ( Epocrates )
|